Background
Clonal cytopenia of undetermined significance (CCUS) is a newly described entity with a high probability to progress into myeloid disorders upon follow-up (Malcovati et al, Blood 2017). Little data is known on how to effectively treat patients with CCUS. We hereby describe a single institution experience in managing symptomatic CCUS patients.
Methods
Patients who had CCUS and underwent active treatment were identified after receiving IRB approval. CCUS diagnosis was rendered if patients had non-diagnostic bone marrow biopsy evaluation combined with evidence of pathogenic myeloid somatic mutation using our institution next generation sequencing (NGS) panel (OncoHeme, Mayo Clinic). "Symptomatic patients" is determined by patients who need therapy beyond blood transfusion. Treatment type is based on physicians' choice. Clinical and lab results were collected. Response and progression were graded based on the MDS IWG 2006 criteria. The date of treatment initiation was used for overall survival (OS) and progression (either to myeloid neoplasm or worsening cytopenias) free survival (PFS) calculation. Stata 14.1(StatCorp) was used for data analysis.
Results
1) Cohort characteristics:
Between 2015 and 2020, 24 patients met inclusion criteria with median age of 72 years (range: 24-87). Twenty (83%) were men. 15 (63%) were white, 1 (4%) African American, 1 (4%) Native American and 7 unknown (33%). Eighty percent had ECOG PS≤1. Four (17%) patients had prior other hematology disorders and 3 (13%) had solid tumor. Two (8%) patients had previous radiation therapy, 2 (8%) received chemotherapy and 1 (4%) received allogeneic stem cell transplantation.
At the time of treatment, the median of hemoglobin was 8.9 (range 6.8-13.7) g/dL, white blood cell 2.9 (range 1.4-12.3) *109/L, and platelet 76 (range 8-407) *109/L. Red blood cell (RBC) and platelet transfusion dependency rate were 54% and 29%, respectively.
We identified 23 different mutations. Most common mutations occurred in SRSF2 (N=5, 21%), TET2 (N=5, 21%), U2AF1 (N=5, 21%), and TP53 (N=4, 17%). Ten (42%) patients had 1 mutation, and 14 (58%) had ≥2 mutations.
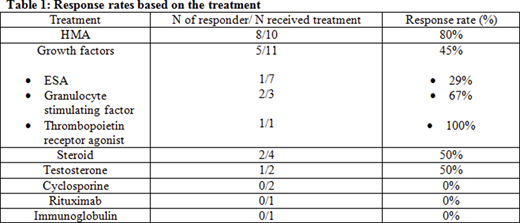
Treatment included hypomethylating agents (HMA) (N=10, 42%), growth factors (N=11, 46%), steroid (N=4, 17%), testosterone (N=2, 8%), cyclosporine (N=2, 8%), rituximab (N=1, 4%), immunoglobulin (N=1, 4%), vitamin B12 and iron (N=1, 4%). Seven (29%) patients received ≥2 treatments. Median follow up duration was 17.5 months (range 8.9-26.1). Median time from the diagnosis to treatment was 2.1 months (range 0-26.8).
2) Molecular correlation with response
The overall response rate (RR) was 50% (hematological or symptomatic improvement). Most responders (67%) were treated with HMA (p=0.013). HMA treatment effect was not significantly associated with DNA methylation genes (DNMT3A, IDH1, IDH2 and TET2, all p>0.05). The RR was 100% for the three patients with IDH1 mutation (treated with HMA, steroid and erythropoietin stimulating agent (ESA), respectively). The RR was 100% for the 3 patients with ZRSR2 mutation (2 HMA, 1 testosterone). The RR was 0% for 3 patients with SF3B1 mutation (1 testosterone and ESA, 1 vitamin B12 and iron, and 1 HMA). The number of mutations had no impact on RR (p=0.41). Table demonstrates the RR for each treatment.
3) Survival and progression outcome
At the last day of follow up, RBC and platelet transfusion dependency rate were 38% and 25%, respectively. The median PFS was 16.9 months (95% CI: 7.5-65.3). Six patients (25%) progressed to myeloid malignancy with 4 myelodysplastic syndrome (MDS) and 2 acute myeloid leukemia (AML). Five patients progressed with worsening cytopenias. Among the 4 MDS patients, they had SF3B1, TET2, and ASXL1 mutations at the diagnosis of CCUS. Within the 2 AML pts, 1 had RUNX1 mutation, 1 had BCOR, DNMT3A, PHF6, RUNX1, SF3B1, STAG2, and TET2 mutations at the diagnosis of CCUS. The median OS was not reached with an estimated 2 year OS 77%. Three (13%) patients progressed with cytopenia and died. Their NGS at CCUS diagnosis showed ASXL1, TP53 and U2AF1 mutations, respectively.
Conclusion
Patients with CCUS responded to available treatments used for myeloid diseases. HMA were effective in this cohort. Mutation profile may have an impact on treatment response. Studies of larger cohorts are needed to validate our findings. Additionally, development of response and progression criteria would be helpful for such patients for reporting treatment outcomes.
Shah:Dren Bio: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal